- Services of Phicell Corporation
- Our Strengths
Strage services for biosamples
We are responsible for the storage and management of samples, specimens, and cells, which require strict procedural and quality control, on behalf of researchers in R&D companies and academia.
We can handle projects as short as one month and as long as 10 years or more for long-term management projects.
Clients can choose from a variety of storage temperature options to suit their storage needs (-150°C zone, -80°C zone, 4°C zone, etc.)
Since we have experienced personnel with backgrounds in research laboratories, we can provide R&D support for biopharmaceuticals and other products with high degree of difficulty.
We can manage specimens not only in bulk by box, but also by single bottle, so we can open storage boxes or pick up a portion of them and ship them out.
We also offer flexible warehousing and shipping operations.
| Storage type | Temperature | Examples |
| Liquid nitrogen cryopreservation container | -150℃ | Cells,Blood,Vaccine etc. |
| Ultra low temperature freezer | -80℃ | DNA,urine etc. |
| Medical cold storage | 4℃ | Investigational Drugs,Specimens etc. |
Transportation

The group company in charge of transportation (SAROUTE Co., Ltd.) has more than 300 vehicles and trained drivers specialized in cell transportation throughout Japan, and has been in the business of transporting cells, culture media, and investigational drugs for over 25 years according to PIC/S GDP and ISO9001 standards.
The company is number one in the industry and has supported many foreign companies in their expansion into Japan.
The company also works with major forwarders FedEx and DHL, and provides support for the preparation of difficult import/export declarations.
The company also has several IATA DG⁽Dangerous Goods Regulations)certified staff.
The company minimizes the effects of the heat wave by using the nighttime hours of Narita International Airport and Kansai International Airport, which operate 24 hours a day, for the loading and unloading of international cargo.
IT service
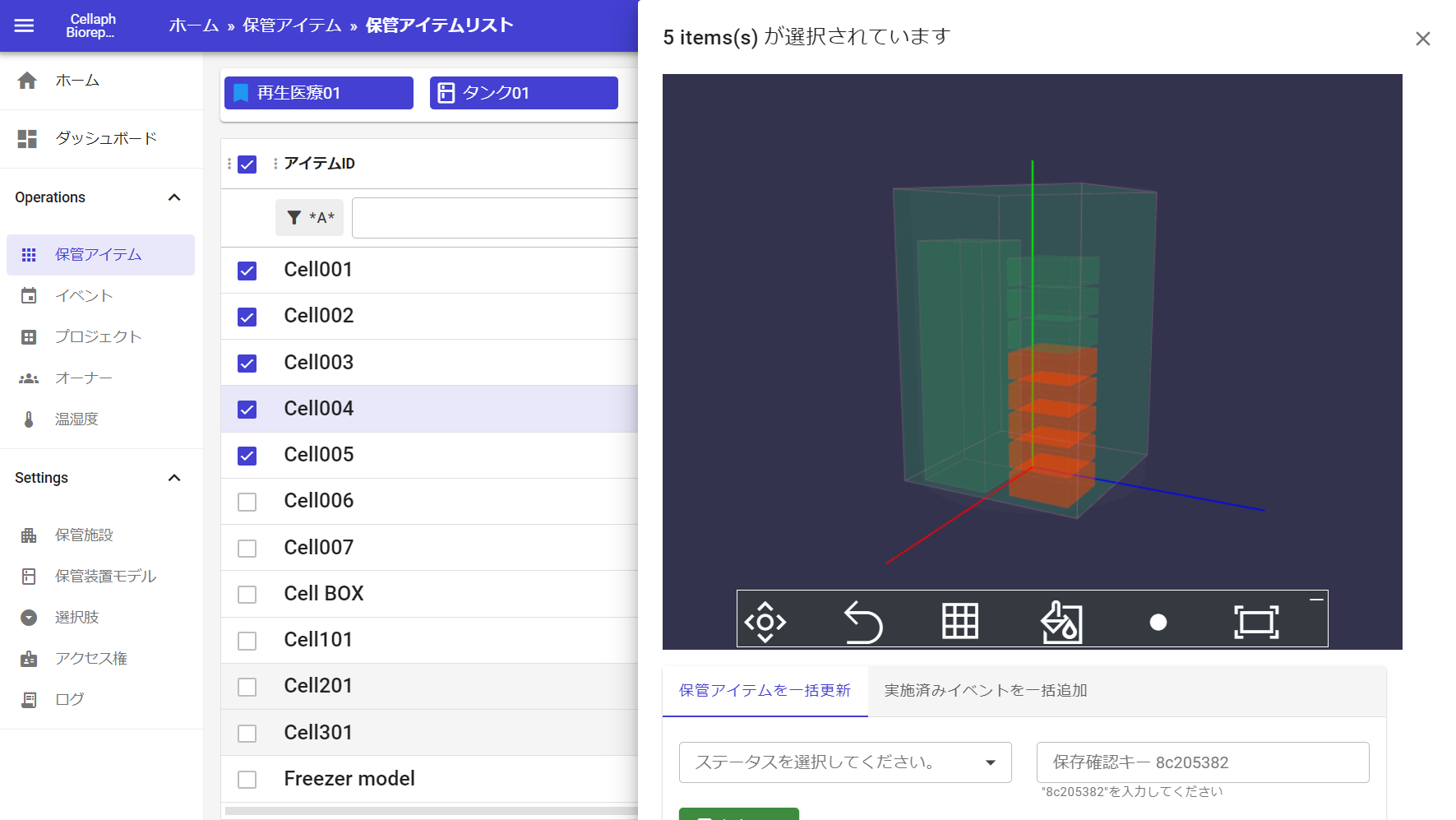
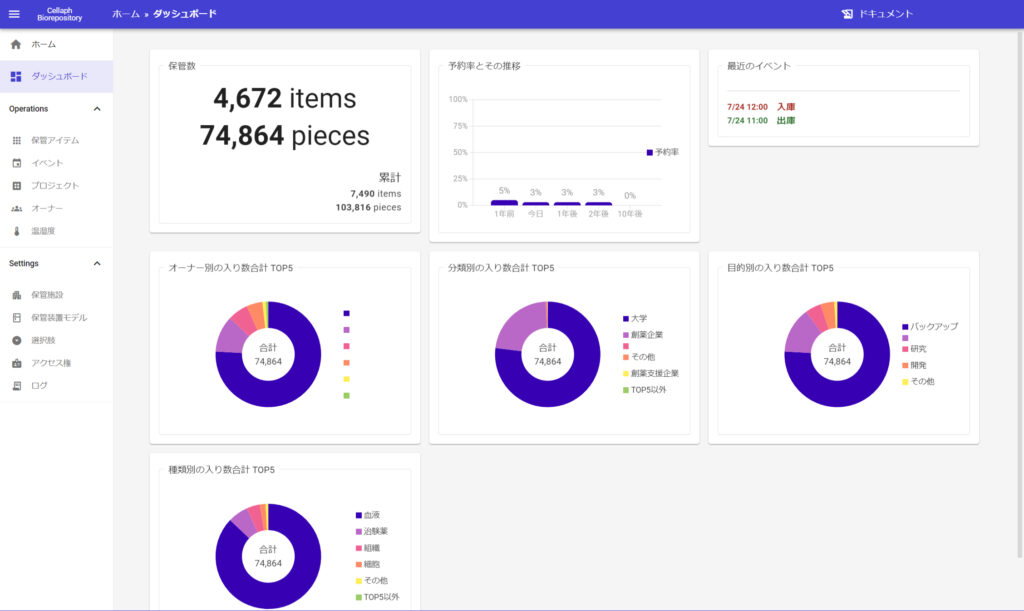
We developed webapp”cellaph” in-house as a sample management system.
”Cellaph”is Japan’s first system* thet allows you to remotely check storagestatus on your browser without actually going to the storage room.
Our system”cellaph” adopting a cloud platform that supports various compliams.
implemented traceability through two-step authentication, device authentication, and activity logs. It is high security and comformity with 21 CFR Part 11.
(*According to our research in November 2019)
Our Strengths
Location
In Japan, where natural disasters are common, we place the highest importance on BCP (Business Continuity Plan) as a requirement for the location of our cell storage facility, as we are entrusted with our clients’ valuable cells.
In 1995, our site was hit by a magnitude 7.3 earthquake in southern Hyogo Prefecture.
As a result of that experience, the site now has liquefaction-resistant ground and tsunami-proof seawalls.
The site’s location, 7 meters above sea level, makes it impervious to hurricane tides, flooding, and storms.
There is also no risk of damage from volcanic eruptions, forest fires, or mudslides.
Above all, one of the world’s most advanced supercomputer “Fugaku,” located right next to the site, is running disaster simulations.
BCP Measures
We are willing to invest in BCP.
As a countermeasure against power outages, the combination of an emergency generator, emergency batteries, and auxiliary cooling cylinders can provide backup for 12 hours or more from the time of trouble until power is restored.
Temperatures in the -150°C, -80°C, and 4°C other storage rooms are monitored in real time by a temperature monitoring system.
Liquid nitrogen tanks for -150°C storage are filled with liquid nitrogen under automatic control.
If the temperature monitoring system alerts the person in charge, the person in charge contacts the 24-hour security office and takes measures according to the emergency manual.
If there is nothing wrong with the building and an individual freezer or cold storage unit breaks down, an auxiliary cooling system is activated in parallel with the alert.
In addition, a large private power generator is also available as a backup power source.
Data security
We would like to emphasize the importance of data security.
Japan is a GDPR (General Data Pataion Regulation) compliant country.
We have a self-developed cell vault management system and use cloud computing, but we intend to continue to limit our use of cloud computing to data centers in Japan.
In addition, all information on the cells we store is anonymized.
Compliance with Quality Standards

Next, let me explain our quality control system.
We are ISO 9001 certified.
We are in the process of applying for a pharmaceutical manufacturing license.
We have documented SOPs in accordance with GMP and have performed IQ (Installation Qualification), OQ (Operation Qualification) and PQ (Performance Qualification) during the installation of facilities and equipment.
We also perform annual OQ and PQ for each storage unit.
I would like to talk about employee training.
New employees spend three months every day learning about GMP, GCP and GDP (Good Distribution Practice) through classroom and on-the-job training.
After that, only those who pass the exam are allowed to work on site.
They then gain experience in cell handling through validation work, periodic report writing, and support roles for veteran employees.
In addition, wet lab workers are trained in manual techniques by veteran employees and are not allowed to work in the field until they pass the training.
They are also required to complete at least 20 hours of continuing education per year, which includes training on important topics such as BCP and emergency preparedness, as well as updates on relevant laws and regulations and trends in the pharmaceutical marketplace.
We also have a complete access control system for our storage facilities.
In addition to 24-hour manned surveillance, no one can enter the facility without going through all the processes, including card, ID and biometric authentication.
Upon entry, visitors change into work clothes and indoor shoes to minimize dust infiltration.
Next to the cell storage room is a cell delivery room, where the storage and delivery areas are clearly separated.
This room is also maintained at a constant temperature and is used for cell packing, checking the temperature log during transport, unpacking the shipping boxes, and cell delivery.
The different procedures for each protocol are communicated in advance between transport and storage personnel to minimize the time cells are exposed to room temperature during the process.
We have also invested in personnel safety in the cell storage room.
Remote monitoring cameras are installed in the cell storage room, and we monitor oxygen levels in the room.
In addition, workers wear fall sensors that alert team members outside the room in the event of a non-environmental fall.
Service Use Cases
Clients use the Company as an outsourcing company for research and development support of pharmaceuticals and regenerative medicine products.
With regard to cell storage, clients may use the service when they want to have distributed storage sites for cells at multiple facilities as a BCP measure.
For transportation, clients can use the Company for the following purposes:
As a collection point prior to overseas export,
As a domestic distribution center (depot) for investigational drugs and cell preparations, etc.
In addition, we support the preparation of specimen storage containers and related materials, such as ID labels to be attached to the storage containers, as an aid to storage consignment.
We also have a wet lab and can handle aliquoting before and after storage.
*Reference information
・Example of cell storage fee
The following is an example of storing 81 containers of 2㎖ each.
The cost would be approximately 70,000 Japanese yen (approximately 482 US dollars at a conversion rate of 145 yen to a dollar) or more.
・Example of transportation charges
The following is an example of a case where 81 containers of 2㎖ each are transported from Kobe to Tokyo, a distance of approximately 500km, by a mix of air and land transportation.
Assuming a temperature condition of -20°C, the cost would be approximately 70,000 Japanese yen (approximately 482 US dollars at a conversion rate of 145 yen to a dollar). However, this excludes options such as the inclusion of a temperature logger.
Track record of transactions
We have 54 cell storage projects in the six years since our founding and more than 93,000 shipments of specimens, biological samples, pharmaceuticals, regenerative medicine products, and investigational drugs annually.
We carefully handle everything from large-scale international clinical trials conducted by Japan’s top three major pharmaceutical companies to small-scale investigator-initiated clinical trials conducted by academia.
We have a proven track record with clients in the corporate, academic and public research sectors, and have been audited by several major pharmaceutical companies, so our procedures are of a high standard.
Contact
If interested, please contact us here.